Invertebrates
Type of resources
Available actions
Topics
Keywords
Contact for the resource
Provided by
Years
Formats
-
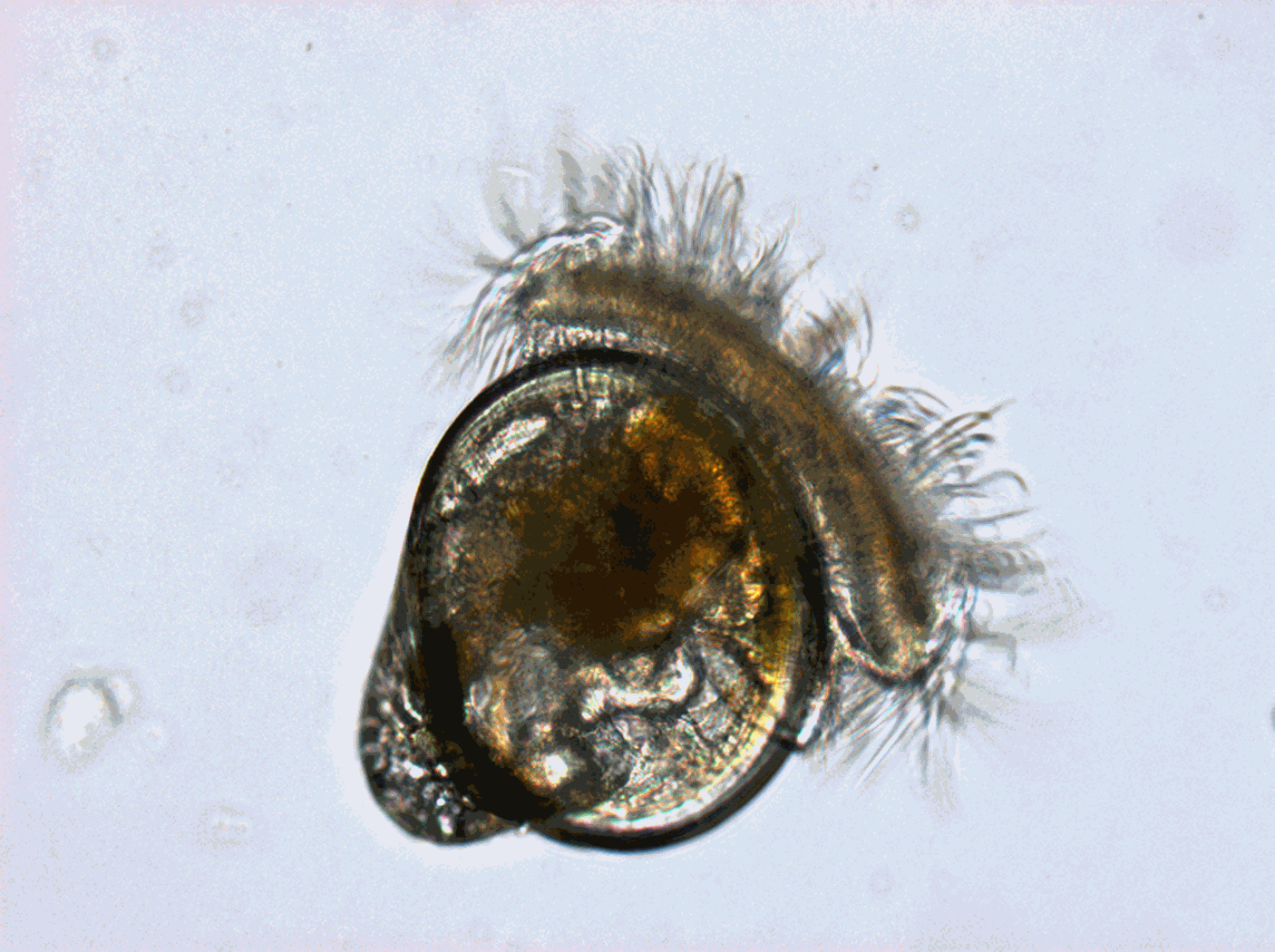
Worldwide, shellfish aquaculture and fisheries in coastal ecosystems represent crucial activities for human feeding. But these biological productions are under the pressure of climate variability and global change. Anticipating the biological processes affected by climate hazards remains a vital objective for species conservation strategies and human activities that rely on. Within marine species, filter feeders like oysters are real key species in coastal ecosystems due to their economic and societal value (fishing and aquaculture) but also due to their ecological importance. Indeed oysters populations in good health play the role of ecosystem engineers that can give many ecosystem services at several scales: building reef habitats that contribute to biodiversity, benthic-pelagic coupling and phytoplankton bloom control through water filtration, living shorelines against coastal erosion… The Pacific oyster, Crassostrea gigas (Thunberg, 1793), which is currently widespread worldwide, was introduced into the Atlantic European coasts at the end of the 19th century for shellfish culture purposes and becomes the main marine species farmed in France (around 100 000 tons) despite severe mortalities crisis. But in the same time and because of warming, natural oysters beds has spread significantly along the French coast and are supposed to have reach approximately 500 000 tons. In that context, Pacific oyster populations (natural and cultivated) in France are the subjects of many scientific projects. Among them, a specific long-term biological monitoring focuses on the reproduction of these populations at a national scale: the VELYGER national program. With more than 8 years of weekly data at many stations in France, this field-monitoring program offers a valuable dataset for studying processes underpinning reproduction cycle of this key-species in relation to environmental parameters, water quality and climate change. Database content: Larval concentration (number of individuals per 1.5 m3) monitored, since 2008, at several stations in six bays of the French coast (from south to north): Thau Lagoon and bays of Arcachon, Marennes Oléron, Bourgneuf, Vilaine and Brest (see map below). Methods used to monitor larval concentration: An important volume of seawater (1.5 m3) is pumped twice a week throughout the spawning season (june-september), at one meter below the surface at high tide (+/- 2h) in several sites within each VELYGER ecosystem. Water is filtered trough plankton net fitted with 40 µm mesh. After a proper rinsing of the net, the retained material is transferred into a polyethylene bottle (1 liter) and fixed with alcohol. At laboratory, sample is then gently filtered and rinse again and transferred into eprouvette. Two sub-samples of 1 mL are then taken using a pipette and examined on a graticule slide for microscope. The microscopic examination is made with a conventional binocular optical microscope with micrometer stage at a magnification of 10 X (or above). During the counting, a special care is necessary as larvae of other bivalves are also collected and confusion is possible. Larvae of C. gigas are also classified into four stage of development: - Stage I = D-shaped straight hinge larvae (shell length <105 µm) - Stage II = Early umbo evolved larvae (shell length between 105 and 150 µm) - Stage III = Medium umbo larvae (shell length between 150 and 235 µm) - Stage IV*= Large umbo eyed pediveliger larvae (shell length > 235 µm) * Larvae that are very closed to settle are sometimes identified into a separated 5th stage, but generally this stage is included in stage IV. Illustrations: Location of the different Velyger sites along the French coast. From south to north: Thau Lagoon and bays of Arcachon, Marennes Oléron, Bourgneuf, Vilaine and Brest. Legend: Pacific Oyster Larvae (left side) and Natural oyster bed (right side). Photos : © S. Pouvreau/Ifremer
-

Survival was recorded at the endpoint for all batches of each group (2n-control, 2n-wild, 2n-commercial, 2nR, 3nR and 3n-commercial). Similarly, initial and final yield were recorded, corresponding to the total weight of the live oysters at deployment and at the endpoint. Finally, shell length and total weight for individually recorded at reception and at the endpoint.
-

The network was initiated by IFREMER from 1993 to 2009 (under the acronym REMORA) to study the rearing performance of the Pacific oyster Crassostrea gigas at a national scale. To do so, the network monitored annually the mortality and growth of standardized batches of 18-month-old oysters. Starting in 1995, the monitoring of the rearing performance of 6-month-old oyster spat was integrated into this network. These sentinel batches were distributed simultaneously each year on 43 sites and were monitored quarterly. These sites were distributed over the main French oyster farming areas and allowed a national coverage of the multiannual evolution of oyster farming performances. Most of the sites were located on the foreshore at comparable levels of immersion. Field studies were carried out by the "Laboratoires Environnement Ressources" (LER) for the sites included in their geographical area of investigation. Following the increase in spat mortality in 2008, the network evolved in 2009 (under the acronym RESCO). From this date, the network selected 13 sites among the 43 sites previously monitored in order to increase the frequency of visits (twice a month) and the number of sentinel batches. More precisely, sentinel batches of oysters corresponding to different origins (wild or hatchery, diploid or triploid) and to two rearing age classes (spat or 18-month-old adults) were selected. The monitoring of environmental variables (temperature, salinity) associated with the 13 sites was also implemented. The actions of the network have thus contributed to disentangle the biotic and abiotic parameters involved in mortality phenomena, taking into account the different compartments (environment / host / infectious agents) likely to interact with the evolution of oyster rearing performance. Finally, since 2015, the network has merged the RESCO and VELYGER networks to adopt the acronym ECOSCOPA. The general objective of this current network is to analyze the causes of spatio-temporal variability of the main life traits (Larval stage - Recruitment - Reproduction - Growth - Survival - Cytogenetic abnormalities) of the cupped oyster in France and to follow their evolution on the long term in the context of climate change. To do this, the network proposes a regular spatio-temporal monitoring of the major proxies of the life cycle of the oyster, organized in three major thematic groups: (1) proxies related to growth, physiological tolerance and survival of experimental sentinel populations over 3 age classes: (2) proxies related to reproduction, larval phase and recruitment of the species throughout its natural range in France, and: (3) proxies related to environmental parameters essential to the species (weather conditions, temperature, salinity, pH, turbidity, chlorophyll a and phytoplankton) at daily or sub-hourly frequencies. Working in a geographical network associating several laboratories, ECOSCOPA provide these monitoring within 8 sites selected among the previous ones to ensure the continuity of the data acquisition. Today, these 8 sites are considered as ecosystems of common interest, contrasted, namely : - The Thau lagoon - The Arcachon basin - The Marennes Oléron basin - The Bourgneuf Bay - The bay of Vilaine - The bay of Brest - The bay of Mont Saint Michel - The bay of Veys The ECOSCOPA network is therefore one of the relevant monitoring tools on a national scale, allowing to objectively measure through different proxies the general state of health of cultivated and wild oyster populations, and this for the different sensitive phases of their life cycle. This network aims at allowing a better evaluation, on the long term, of the biological risks incurred by the sector but also by the ecosystems, in particular under the increasing constraint of climatic and anthropic changes. Figure : Sites monitored by the ECOSCOPA network
-
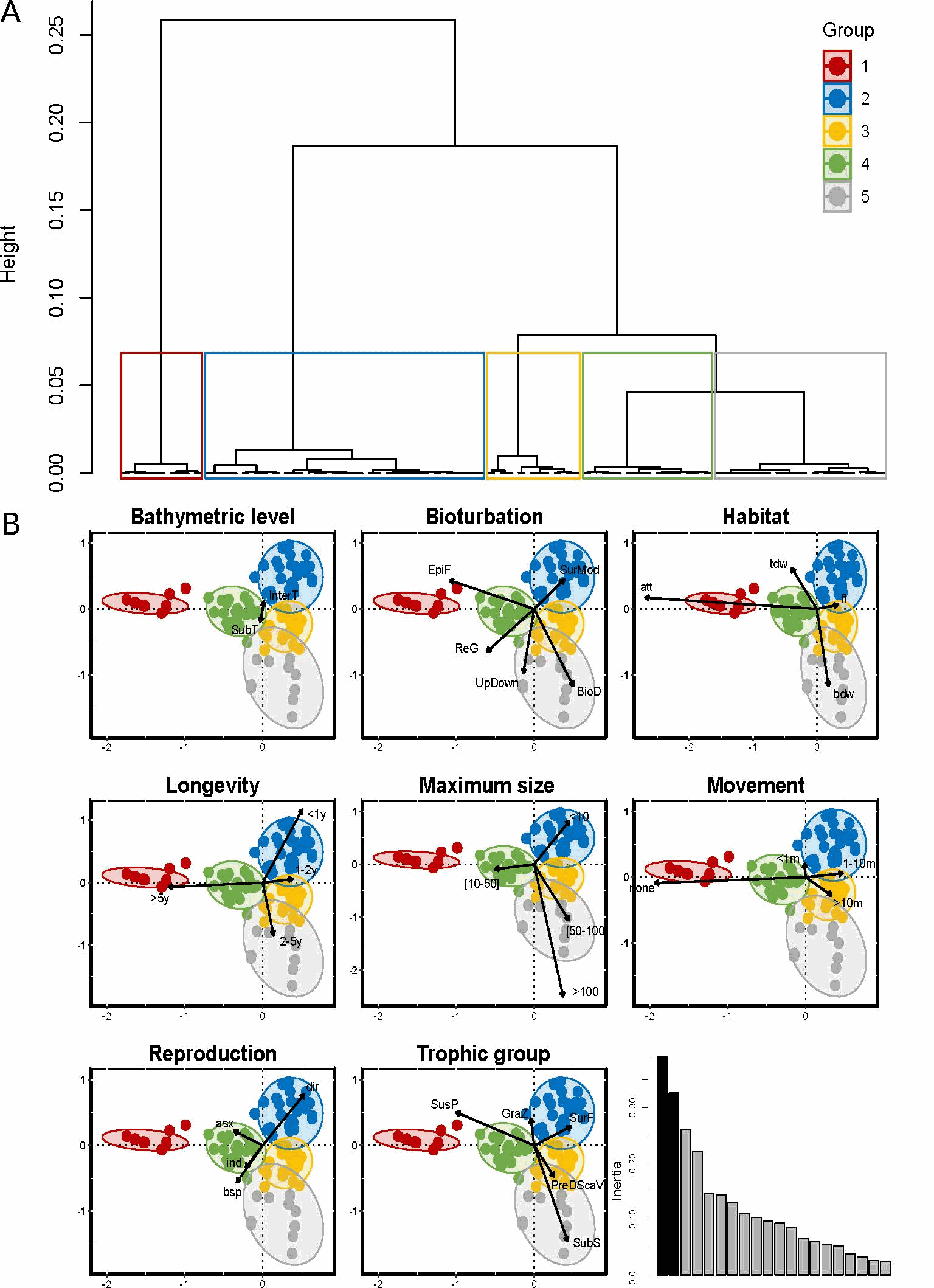
Reef-building species are recognized as having an important ecological role and as generally enhancing the diversity of benthic organisms in marine habitats. However, although these ecosystem engineers have a facilitating role for some species, they may exclude or compete with others. The honeycomb worm Sabellaria alveolata (Linnaeus, 1767) is an important foundation species, commonly found from northwest Ireland to northern Mauritania (Curd et al., 2020), whose reef structures increase the physical complexity of the marine benthos, supporting high levels of biodiversity. Local patterns and regional differences in taxonomic and functional diversity were examined in honeycomb worm reefs from ten sites along the northeastern Atlantic to explore variation in diversity across biogeographic regions and the potential effects of environmental drivers. To characterize the functional diversity at each site, a biological trait analysis (BTA) was conducted (Statzner et al., 1994). Here we present the functional trait database used for the benthic macrofauna found to live in association with honeycomb worm reefs. Eight biological traits (divided into 32 modalities) were selected (Table 1), providing information linked to the ecological functions performed by the associated macrofauna. The selected traits provide information on: (i) resource use and availability (by the trophic group of species, e.g. Thrush et al. 2006); (ii) secondary production and the amount of energy and organic matter (OM) produced based on the life cycle of the organisms (including longevity, maximum size and mode of reproduction, e.g. (Cusson and Bourget, 2005; Thrush et al., 2006) and; (iii) the behavior of the species in general [i.e. how these species occupy the environment and contribute to biogeochemical fluxes through habitat, movement, and bioturbation activity at different bathymetric levels, e.g. (Solan et al., 2004; Thrush et al., 2006; Queirós et al., 2013). Species were scored for each trait modality based on their affinity using a fuzzy coding approach (Chevenet et al., 1994), where multiple modalities can be attributed to a species if appropriate, and allowed for the incorporation of intraspecific variability in trait expression. The information concerning polychaetes was derived primarily from Fauchald et al (1979) and Jumars et al (2015). Information on other taxonomic groups was obtained either from databases of biological traits (www.marlin.ac.uk/biotic) or publications (Naylor, 1972; King, 1974; Caine, 1977; Lincoln, 1979; Holdich and Jones, 1983; Smaldon et al., 1993; Ingle, 1996; San Martín, 2003; Southward, 2008; Gil, 2011; Leblanc et al., 2011; Rumbold et al., 2012; San Martín and Worsfold, 2015; Jones et al., 2018). Map indicating the locations of the 10 study sites in the UK, France and Portugal within the four biogeographic provinces defined by Dinter (2001). (All sites were sampled in 8 different stations, except for UK4 where 5 stations were sampled).
-

The Mytilobs network, carried out by IFREMER (French Research Institute for Exploitation of the Sea), is a national network dedicated to building long-term physiological variations time series of blue mussels (Mytilus edulis), across a large spatial scale. This observation network, initially designed to survey production yields, also provides valuable data to track environmental variations of coastal ecosystems. Mussels exhibit high phenotypic plasticity in response to environmental variations. Collection of data describing phenotypic variations, over an extended period, reveals small-scale climate and habitat variations. With its broad deployment across time and space, the data produced under Mytilobs will be useful for the establishment of a baseline condition when studying the effect of a perturbation affecting an ecosystem’s functioning. Finally, the monitoring of mussel biometric traits and mortality was coupled with high-frequency measurements of salinity, temperature, and sea level, complementing this multi-layer observational framework.
-

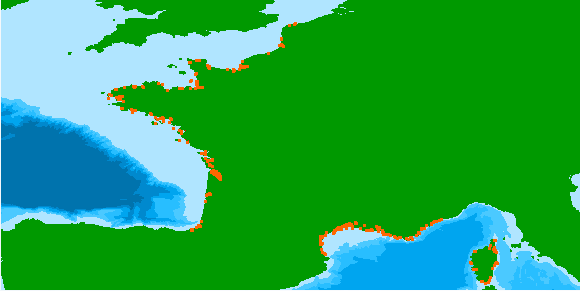
Numerous reef-forming species have declined dramatically in the last century, many of which have been insufficiently documented due to anecdotal or hard-to-access information. One of them, the honeycomb worm Sabellaria alveolata (L.) is a tube-building polychaete that can form large reefs, providing important ecosystem services such as coastal protection and habitat provision. It ranges from Scotland to Morocco, yet little is known about its distribution outside of the United Kingdom, where it is protected and where there is a strong heritage of natural history and sustained observations. As a result, online marine biodiversity information systems currently contain haphazardly distributed records of S. alveolata. One of the objectives of the REEHAB project (http://www.honeycombworms.org) was to combine historical records with contemporary data to document changes in the distribution and abundance of S. alveolata. Here we publish the result of the curation of 446 sources, gathered from literature, targeted surveys, local conservation reports, museum specimens, personal communications by authors and by their research teams, national biodiversity information systems (i.e. the UK National Biodiversity Network (NBN), https://nbn.org.uk/) and validated citizen science observations (i.e. https://www.inaturalist.org/). 80%[ar1] of these records were not previously referenced in any online information system. Additionally, historic field notebooks from Edouard Fischer-Piette and Gustave Gilson were scanned for S. alveolata information and manually entered. The original taxonomic identification of the 23296 S. alveolata records has been kept. Some identification errors may however be present, particularly in the English Channel and the North Sea where incorrectly identified observations of intertidal Sabellaria spinulosa were recorded. A further 229 observations are recorded as ‘Sabellaria spp.’ as the available information does not allow a species-level identification. Many sources reported abundances based on the semi-quantitative SACFOR scale while others simply noted its presence, and others still verified both its absence and presence. The result is a curated and comprehensive dataset spanning over two centuries on the past and present global distribution and abundance of S. alveolata. Sabellaria alveolata records projected onto a 50km grid. When SACFOR scale abundance scores were given to occurrence records, the highest abundance value per grid cell was retained.
-
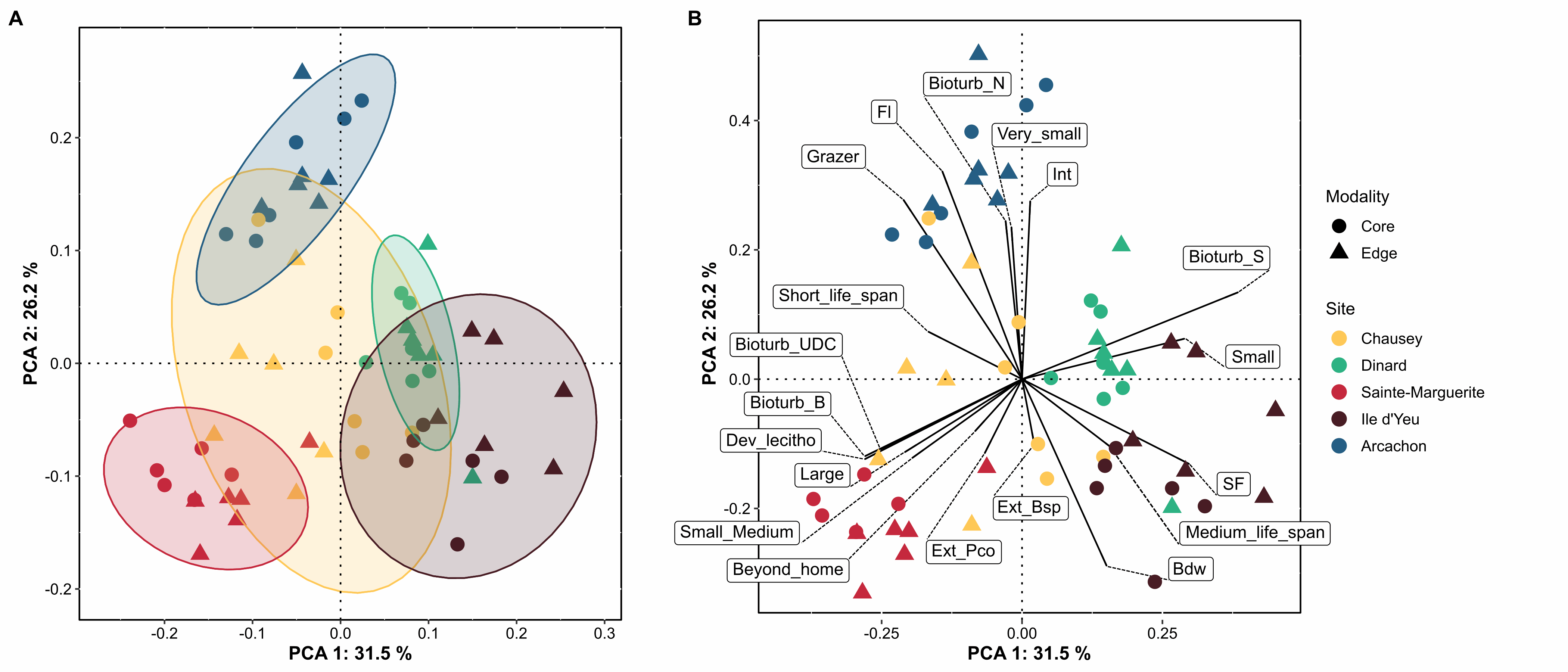
Zostera marina (Linnaeus, 1753) is a flowering marine plant that occurs from temperate to subantarctic regions (Green and Short, 2003), forming meadows that are recognized as being among the most important ecosystems on the planet (Costanza et al., 1997; Duffy, 2006; Duarte et al., 2008; Dewsbury et al., 2016). Eelgrass is a foundation species, providing essential functions and services including coastal protection, erosion control, nutrient cycling, water purification, carbon sequestration, as well as food and habitat for a variety of species (Duarte 2002; Heck et al. 2003; Healey & Hovel 2004, Orth et al. 2006; Barbier et al., 2011; Fourqurean et al. 2012; Cullen-Unsworth & Unsworth 2013; Schmidt et al. 2011, 2016). Eelgrass can have a strong influence on the spatial distribution of associated fauna by altering the hydrodynamics of the marine environment (Fonseca and Fisher 1986), stabilizing sediments (Orth et al. 2006), providing abundant resources, available surface area, and increased ecological niches. Meadows also provide protection from predation by providing greater habitat complexity both above and below ground (Heck and Wetstone 1977; Orth et al. 1984; Gartner et al. 2013, Reynolds et al., 2018). Local patterns and regional differences in the taxonomic and functional diversity of assemblages associated with five Zostera marina meadows occurring over a distance of 800 km along the coast of France were investigated with the objective of determining which factors control community composition within this habitat. To this end, we examined - and -diversity of species- and trait-based descriptors, focused on polychaetes; bivalves and gastropods, three diverse groups exhibiting a wide range of ecological strategies (Jumars, Dorgan, & Lindsay, 2015) and having central roles in ecosystem functioning through activities such as bioturbation or trophic regime (Queirós et al., 2013, Duffy et al., 2015). Here we present the abundance (Table 1) and the functional trait database (Table 2) used for the benthic macrofauna found to live in association with eelgrass meadows in Chausey, Dinard, Sainte-Marguerite, Ile d’Yeu and Arcachon, sampled in the fall of 2019. Eight biological traits (divided into 32 modalities, Table S1) were selected, providing information linked to the ecological functions performed by the associated macrofauna. The selected traits provide information on: (i) resource use and availability (by the trophic group of species, e.g. Thrush et al. 2006); (ii) secondary production and the amount of energy and organic matter (OM) produced based on the life cycle of the organisms (including longevity, maximum size and mode of reproduction, e.g. (Cusson and Bourget, 2005; Thrush et al., 2006) and; (iii) the behavior of the species in general [i.e. how these species occupy the environment and contribute to biogeochemical fluxes through habitat, movement, and bioturbation activity, e.g. (Solan et al., 2004; Thrush et al., 2006; Queirós et al., 2013). Species were scored for each trait modality based on their affinity using a fuzzy coding approach (Chevenet et al., 1994), where multiple modalities can be attributed to a species if appropriate, and allowed for the incorporation of intraspecific variability in trait expression. Information for polychaetes was primarily extracted from Fauchald et al (1979), Jumars et al (2015), and Boyé et al (2019). Information for mollusks was obtained either from biological trait databases (www.marlin.ac.uk/biotic, www.univie.ac.at/arctictraits, Bacouillard et al 2020) or from publications (e.g. Queiros et al. 2013; Thrush et al, 2006; Caine, 1977). Information was collected at the lowest possible taxonomic level and when missing was based on data available in other species of the genus, or in some cases, in the same family (only for traits with low variability for these families). Figure 1. Map indicating the locations of the 5 study sites of Zostera marina meadows in France: three in the the English Channel, and two in the Bay of Biscay (all sites were sampled in 6 different stations).
-
French benthic invertebrates composition and abundance taxa data are collected during monitoring surveys on the English Channel / Bay of Biscay coasts and Mediterranean coast (Quadrige program code : REBENT_FAU, RSL_FAU). Protocols are implemented in the Water Framework Directive. Data are transmitted in a Seadatanet format (CDI + ODV) to EMODnet Biology european database. 498 ODV files have been generated from period 01/01/2003 to 31/12/2021.
-
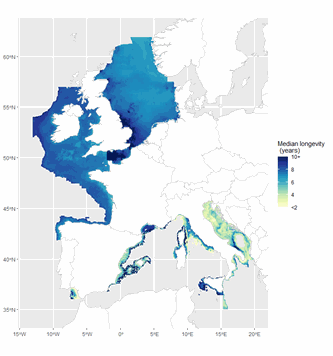
The ICES Working Group on Fisheries Benthic Impact and Trade-offs (WGFBIT) has developed an assessment framework based on the life history trait longevity, to evaluate the benthic impact of fisheries at the regional scale. In order to apply this framework to the Mediterranean sea, several Mediterranean longevity databases were merged together with existing North-East Atlantic ones to develop a common database. Longevity was fuzzy coded into four longevity classes: <1, 1-3, 3-10 and >10 years. Both benthic mega and macrofauna organisms are included in this dataset. Further details about both the purpose and the methodology may be found in ICES (2022) and Cuyvers et al. (2023). The result of the final dataset merging is one dataset containing the fuzzy coded average longevity (and standard deviation) for 2264 taxa and for each, the number of databases used.
-

Ifremer conducts numerous fisheries surveys dedicated to benthic and demersal populations (commercial / non-commercial fishes and invertebrates). For several years, in application of the ecosystem approach, all benthic invertebrate fauna collected in fishing gear has been systematically monitored: megabenthic invertebrates captured have been sorted, identified, counted and weighted. All these surveys are based on fixed or random stratified sampling strategy with varying intensity depending on the covered survey area. These data are stored, in historical access-based databases or for the most recent years in the centralised “Harmonie” database held in the Ifremer Fishery Information Systeme (SIH). The species nomenclature used was standardized using WoRMS database. Taxa caught at least once a year are listed for each monitoring area on the basis of already available data series. In order to facilitate the identification of individuals sampled on board vessels and to improve the training of onboard scientists, the present work aims to define the minimum level of identification for each of them. The analysis identifies taxa that appears recurrently on available historical series or gathers them on less precise taxonomic levels if this is not the case, which may indicate potential identification difficulties. The following procedure was used: all taxa expressed at the species level were first aggregated at genus level if they occurred less 90% of the years over the available time series. For MEDITS, EPIBENGOL and ORHAGO, the occurrence threshold was set to 70% and to only 50% for NOURMONT because the datasets were less than 10 years long. Then to be kept at that taxonomic level, a given genus had to be observed over 90% of the time (for example over at least 9 years if the dataset contains 10 years). Otherwise it was iteratively regrouped into a higher taxonomic level (family, order, class, division) following the same criteria (Foveau et al, 2017). For instance, for the NOURSEINE survey, this resulted into the aggregation of the 103 origin taxa into 35 taxonomic groups. The name of the final taxon after data processing represents the minimum level of identification defined by the analysis. However, these results are very theoretical. This is why they were sent to scientists who embark regularly in order to refine the level of taxonomic identification with field experience. The first dataset is composed of 8 tables relevant to the different vessel surveys. The first column of each table represents the permanent code of the taxon in the Ifremer taxonomic referential, the second the systematic number and the third the species abbreviated code. The other columns are the different taxonomic levels of the taxon. The minimum level of identification at sea defined by the data processing appears in blue. The level determined by feedback of scientist’s field experience, which is the one to use at sea, appears in green. The second dataset summaries the results detailed in the first table and indicates directly for each taxon identified to far, the minimum level of identification required for the benthic invertebrates by-catch of each fisheries surveys studied.
 Catalogue PIGMA
Catalogue PIGMA